with PAH receiving PDE5i do not reach or
maintain treatment goals
Study rationale
Clinical data indicate that 20–60% of patients with PAHPAH, pulmonary arterial hypertension receiving PDE5iPDE5i: phosphodiesterase type 5 inhibitor do not reach or maintain treatment goals.1, 2, 3 Escalation of therapy, including combination therapy, is an option in these patients4, 5; however, combination therapy may have additional side effects,6 and important financial considerations.7, 8

- Riociguat, an sGCsGC, soluble guanylate cyclase stimulator, acts on the same molecular pathway as PDE5iPDE5i: phosphodiesterase type 5 inhibitor, the NONO, nitric oxide– sGCsGC, soluble guanylate cyclase– cGMPcGMP, cyclic guanosine monophosphate pathway, but has a different mode of action and molecular target within the pathway.
- In healthy subjects, activation of sGCsGC, soluble guanylate cyclase by NONO, nitric oxide results in production of cGMPcGMP, cyclic guanosine monophosphate, leading to vasodilation in the pulmonary circulation.9 PDE5 degrades cGMPcGMP, cyclic guanosine monophosphate; thus, PDE5iPDE5i: phosphodiesterase type 5 inhibitor increase intracellular cGMPcGMP, cyclic guanosine monophosphate levels.9
- However, in patients with PAHPAH, pulmonary arterial hypertension, NONO, nitric oxide bioavailability is reduced,10, 11, 12, 13, 14 which may render PDE5iPDE5i: phosphodiesterase type 5 inhibitor ineffective. Unlike PDE5iPDE5i: phosphodiesterase type 5 inhibitor, riociguat directly stimulates sGCsGC, soluble guanylate cyclase, increasing intracellular cGMPcGMP, cyclic guanosine monophosphate independently of NONO, nitric oxide.15, 16
- Riociguat may therefore be effective in patients with PAHPAH, pulmonary arterial hypertension who do not respond adequately to PDE5iPDE5i: phosphodiesterase type 5 inhibitor through optimization of the NONO, nitric oxide–sGCsGC, soluble guanylate cyclase–cGMPcGMP, cyclic guanosine monophosphate pathway.
- The uncontrolled, open-label RESPITE pilot study investigated switching therapies that target the same signaling pathway.17 Switching to riociguat was associated with improvements from baseline in 6MWD6MWD, 6-minute walking distance, NT-proBNPNT-proBNP, N-terminal prohormone of brain natriuretic peptide, and WHO FCWHO FC, World Health Organization functional class.
- Given the positive trends seen in RESPITE, further data were required to confirm that patients with PAHPAH, pulmonary arterial hypertension on stable PDE5iPDE5i: phosphodiesterase type 5 inhibitor therapy but not at treatment goal can achieve clinical improvement by switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor to riociguat.
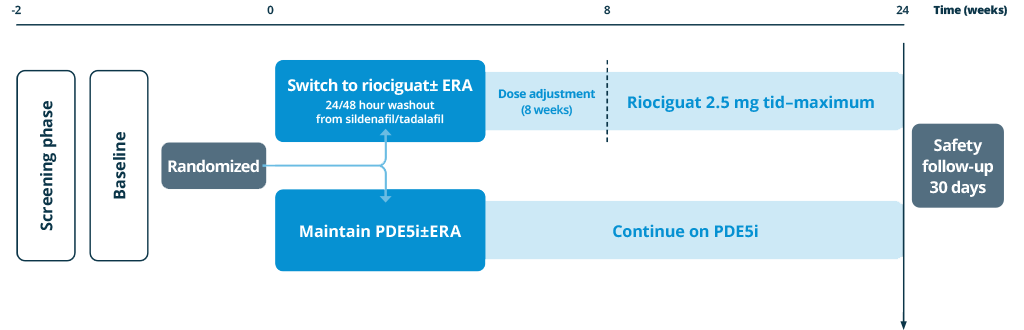
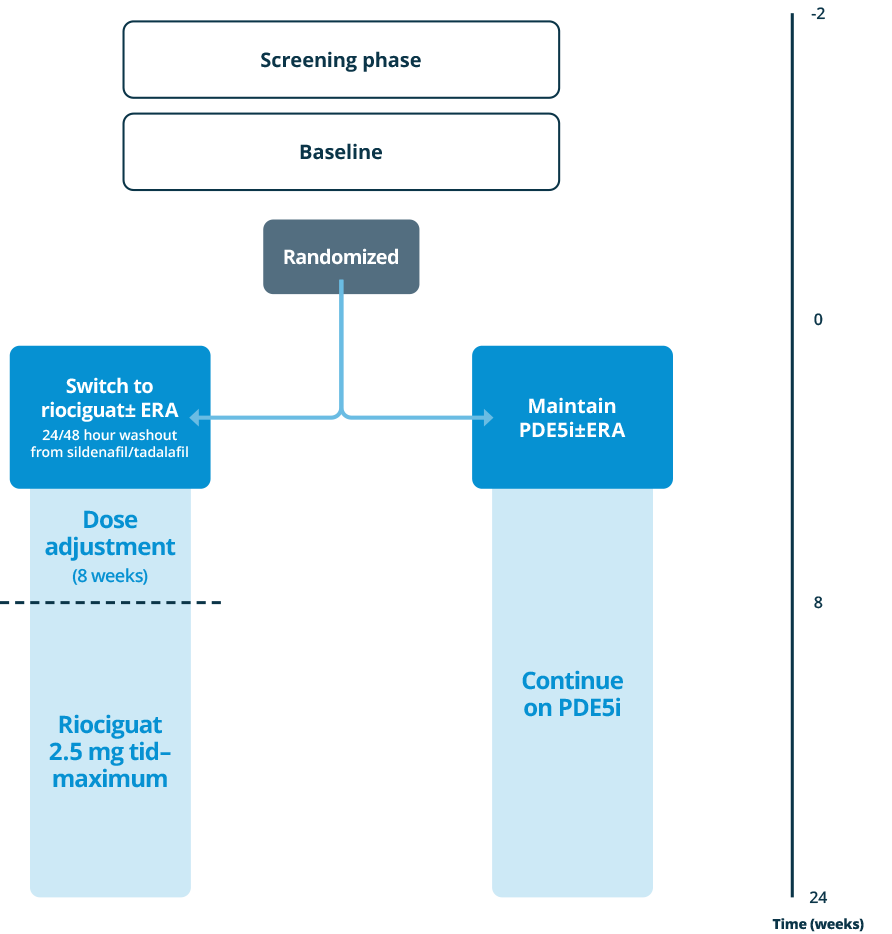
- The prospective, randomized, controlled REPLACE study investigated the efficacy and safety of switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor (±ERAERA, endothelin receptor antagonist background therapy) compared with remaining on PDE5iPDE5i: phosphodiesterase type 5 inhibitor.18
Patient population
Patients treated with a stable dose of PDE5iPDE5i: phosphodiesterase type 5 inhibitor±ERAERA, endothelin receptor antagonist for 5 weeks, at intermediate risk
Intermediate risk, as indicated by:
- WHO FCWHO FC, World Health Organization functional class III
- 6MWD6MWD, 6-minute walking distance 165-440 m
Primary endpoint (centrally adjudicated)
- Clinical improvement at Week 24, defined as 2 out of 3:
- 6MWD6MWD, 6-minute walking distance improvements by ≥ 10% or ≥ 30 m
- WHO FCWHO FC, World Health Organization functional class I/II
- NT-proBNPNT-proBNP, N-terminal prohormone of brain natriuretic peptide decrease by ≥ 30%
- Absence of clinical worsening
Study sites
The REPLACE study was conducted in 81 centers in 22 countries
[interactive-map]
Resources

Find resources associated with the study, including other riociguat publications
- Chockalingam A et al. Int J Cardiol 2005;99:91–95.
- Leuchte HH et al. Chest 2004;125:580–586.
- Galiè N et al. N Engl J Med 2015;373:834–844.
- Galiè N et al. Eur Respir J 2015;46:903–975.
- Lajoie AC et al. Lancet Respir Med 2016;4:291–305.
- Liu HL et al. Chest 2016;150:353–366.
- Hill NS et al. J Manag Care Spec Pharm 2016;22:S3–S21.
- Wilkens H et al. Respir Med 2010;104:902–910.
- Ghofrani HA et al. Nat Rev Drug Discov 2006;5:689–702.
- Archer SL et al. Am J Respir Crit Care Med 1998;158:1061–1067.
- Giaid A and Saleh S. N Engl J Med 1995;333:214–221.
- Kielsten JT et al. Arterioscler Thromb Vasc Biol 2005;25:1414–1418.
- Migneault A et al. Am J Respir Crit Care Med 2005;171:506–513.
- Tsai EJ and Kass DA. Pharmcol Ther 2009;122:216–238.
- Stasch JP et al. Nature 2001;410:212–215.
- Stasch JP et al. Circulation 2011;123:2263–2273.
- Hoeper MM et al. Eur Respir J 2017;50:1602425.
- Hoeper, M. M et al. Am J Respir Crit Care Med 195: A2296.