Click here to view the publication


The REPLACE study results
The REPLACE study met its primary endpoint. Switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor to riociguat resulted in a significantly higher proportion of patients experiencing clinical improvement and significantly lower rates of clinical worsening compared with remaining on PDE5iPDE5i: phosphodiesterase type 5 inhibitor.

The REPLACE study results presentation
On behalf of the REPLACE investigators, Professor Marius Hoeper presents the findings of REPLACE.
In this video summary, Professor Hoeper discusses the study rationale and design, results data, and clinical implications of the study.
Overview of key findings
Patients switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor to riociguat had a significantly higher likelihood of clinical improvement and significantly reduced rate of clinical worsening compared with patients remaining on PDE5iPDE5i: phosphodiesterase type 5 inhibitor
Primary endpoint
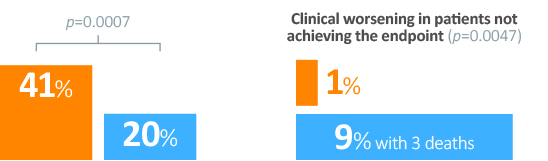
Secondary endpointsa

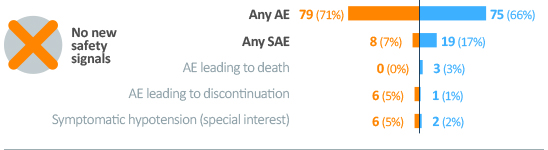
Safety
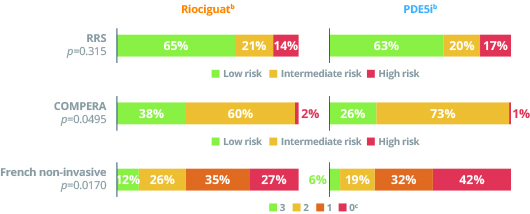
Risk profiles at Week 24
• REPLACE is the first randomized, controlled study in patients with PAHPAH, pulmonary arterial hypertension investigating switching within the same pathway, and also the first head-to-head RCTRCT, randomized controlled trial of approved PAHPAH, pulmonary arterial hypertension therapies
• Riociguat was generally well tolerated in patients who switched from PDE5iPDE5i: phosphodiesterase type 5 inhibitor. Overall AEAE, adverse event rates were similar between the treatment groups, with a higher incidence of SAEsSAE, serious adverse event in the PDE5iPDE5i: phosphodiesterase type 5 inhibitor group
• REPLACE demonstrated that switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor (± ERA) to riociguat can benefit patients with PAHPAH, pulmonary arterial hypertension at intermediate risk and can serve as a strategic option for treatment escalation
• By optimizing the NONO, nitric oxide–sGCsGC, soluble guanylate cyclase–cGMPcGMP, cyclic guanosine monophosphate pathway by switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor to riociguat, patients can remain on monotherapy or dual combination therapy, delaying the addition of further therapies to a later stage
• Riociguat was generally well tolerated in patients who switched from PDE5iPDE5i: phosphodiesterase type 5 inhibitor. Overall AEAE, adverse event rates were similar between the treatment groups, with a higher incidence of SAEsSAE, serious adverse event in the PDE5iPDE5i: phosphodiesterase type 5 inhibitor group
• REPLACE demonstrated that switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor (± ERA) to riociguat can benefit patients with PAHPAH, pulmonary arterial hypertension at intermediate risk and can serve as a strategic option for treatment escalation
• By optimizing the NONO, nitric oxide–sGCsGC, soluble guanylate cyclase–cGMPcGMP, cyclic guanosine monophosphate pathway by switching from PDE5iPDE5i: phosphodiesterase type 5 inhibitor to riociguat, patients can remain on monotherapy or dual combination therapy, delaying the addition of further therapies to a later stage
aSecondary endpoints were not powered to show an effect.
bThe risk profiles for the patient groups were similar at baseline.
cThe French non-invasive scale refers to the number of parameters in the low risk range.
bThe risk profiles for the patient groups were similar at baseline.
cThe French non-invasive scale refers to the number of parameters in the low risk range.
Resources

Find resources associated with the study, including other riociguat publications
Hoeper MM et al Eur Respir J 2020 Vol 56 suppl 63, e-poster presented at the 2020 European Respiratory Society Virtual Congress